Azeotropic Distillation Process
Azeotropic Distillation Process:– Distillation is a process in which a liquid mixture of volatile components is separated by imparting energy to it in consideration with the boiling points of the components so that selective vaporization takes place. Whenever vapours of a mixture are produced the vapours are relatively richer in more volatile components compared to the liquid mixture. It means the composition of the more volatile components in the vapour phase have higher values than the more volatile components present in the liquid phase.
At times, for some selection of components to form the liquid mixture, while boiling the liquid mixture a point may reach when we see that the value of composition of each component in the vapour phase will be equal to the value of the composition of the each same component in the liquid phase. When such mixtures will be boiled further they will not produce any change in the composition of the components in vapour or liquid phase, meaning the compositions will remain constant. Such mixtures are called Azeotropes. The azeotropes can’t be separated by simple distillation process.
what is Azeotropic Distillation ?
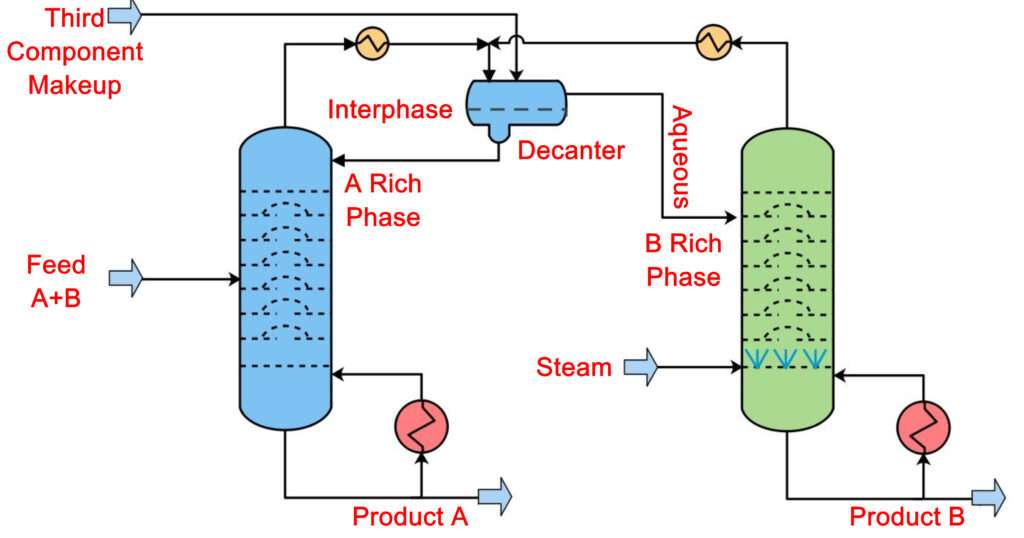
Azeotropic distillation techniques are range of techniques which are used to separate the azeotrope in a distillation column. Most commonly this is done by adding a foreign material into the azeotropic mixture. Often Pressure swing technique is used and also sometimes reactive distillation is used in order to separate an azeotrope.
Adding a Material to the Mixture
The azeotropes can be either of two types; minimum boiling azeotropes or maximum boiling azeotropes. Minimum boiling azeotropes show positive deviations from raoult’s law and maximum boiling azeotropes show negative deviations from raoult’s law. Minimum boiling azeotropes boil at relatively low temperatures and maximum boiling azeotropes boil at relatively high temperature.
What is Azeotropic Mixture ?
In azeotropic distillation, a foreign material is added to the azeotropic mixture. This material is called an entrainer and usually it is used in small quantities. The entrainer is specifically selected to be of such nature that when it interacts with the azeotrope mixture it will alter it such that the entrainer will form a new azeotrope, a low boiling azeotrope with one of the component of the azeotrope.
It means when the mixture will be boiled then this newly formed low boiling azeotrope will move out of the system from the top of the column and we will recover one of the components of the original azeotrope as a bottoms product. This is the defining feature of azeotropic distillation.
The choice of entrainer is very important because only a properly selected entrainer can alter the molecular interactions of the azeotrope in order to eliminate it and will form a new low boiling azeotrope with one of the components based on the polarity.
The entrainer selected must have the same volatility as the original azeotropic mixture to be separated. The entrainer must be non-toxic, non-flammable for safety concerns. It is preferable of it is cheap and easily available. Most importantly the entrainer selected must be easily recoverable in order for the process to be economical.
Reference:- WIKI
































