Urea Manufacturing Process
Urea Manufacturing Process
Urea is an organic chemical compound which is also referred sometimes as Carbamide. It is usually manufactured to be stored as a solid, commonly in shape and form of prills and granules. These urea granules are highly soluble in water hence it is easier to form their solutions at varying concentration. These solutions can also be stored if required. It has a very high nitrogen content.
Urea is naturally produced in human body and disposed as a natural cyclic process of excretion by Kidneys. Urea helps carry waste nitrogen out of the body. Historically urea is a significant chemical compound because it was the first organic chemical which is naturally found in human body to be synthesized using inorganic chemicals in a laboratory by Friedrich Wöhler. Due to this achievement some people refer to him as father of organic chemistry.
Urea is used in Textile, Plastic, Adhesive, Coatings industries. The most popular and most common use of urea is as a fertilizer not only because it has the highest nitrogen content of 46% compared to other fertilizers but also because it dissolves readily in water and leaves no salt residues on the soil and crops. Also, it can be used for foliar feeding. The major disadvantage of using urea as a fertilizer is that it may contain a chemical compound named biuret which is formed during manufacturing of urea. If the biuret concentration exceeds 2% then it is harmful to plants especially germinating seeds and also is toxic to animals. Controlling the concentration by optimizing equipment design and temperature is a major industrial problem in urea manufacturing which demands continuous research.
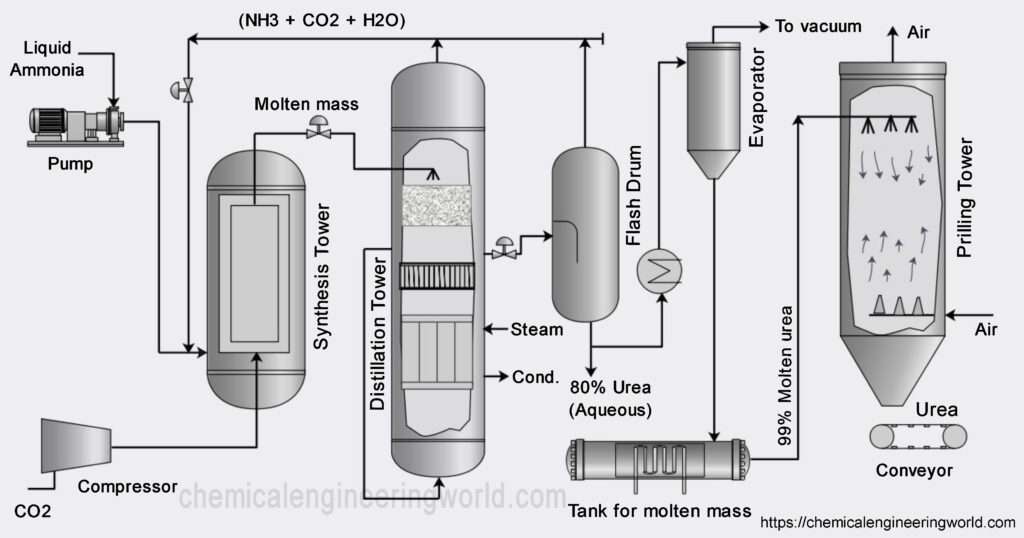
Urea Manufacturing Process
The raw materials which are used in urea manufacturing process are ammonia and carbon dioxide hence generally urea is manufactured in an ammonia plant because it yields ammonia as a product and carbon dioxide as a byproduct and this carbon dioxide can be used directly for manufacturing urea.
Two chemical reactions are primarily involved in the process:
- Reaction of Ammonia and Carbon Dioxide to form Ammonium Carbamate.
2NH3 + CO2 → NH2COONH4
- Decomposition of Ammonium Carbonate to form Urea and Water.
NH2COONH4 → H2O + NH2CONH2 (urea)
Note:- Above both reaction are reversible
Liquid ammonia is pumped and carbon dioxide is compressed and transported to an equipment called reaction chamber. Since this is were the reaction happens, it is the heart of the process. The pressure and temperature is maintained at 14 Mpa and 170-190°C for the first reaction to occur. The reaction of ammonia and carbon dioxide is highly exothermic in nature. Most of the heat released is utilized in form of process steam wherever it is needed in to process.
The product from the first reaction flow into a decomposer where the second reaction occurs and it is endothermic reaction. It requires certain energy to begin. Biuret is also formed as a result of decomposition of ammonium carbamate if temperature rise is excessive.
The conversion of the reactants to urea can be increased by increasing the amount of carbon dioxide, if carbon dioxide is present in excess then the conversion can be as high as 85% per pass but optimizing for the proper temperature, pressure and design is a challenge in itself hence usually per pass conversion are kept around 50%. The unreacted materials are recycled resulting in overall conversion of over 99%. This minimises the effects on environment.
The major impurity in urea is water and also unreacted ammonia, carbon dioxide and ammonium carbamate. These are removed using distillation tower and evaporator. Essential condition is to keep temperature high and pressure low during stages of separation. At these conditions the ammonium carbamate will be decomposed back to ammonia and carbon dioxide also some carbon dioxide and ammonia will flash off. The major process which happens in the evaporator is that of concentration. During concentration optimum temperature should be maintained so that the urea remains in molten state and crystals are not formed inside the evaporator.
The molten urea is passed through nozzles inside the prilling tower. Compressed air is passed in the tower so that its flow is counter current with respect to that of molten urea. The urea gets solidified in the prilling tower and air helps in shaping it in the form of prills or granules. The urea is then stored and ready to be sold.
































