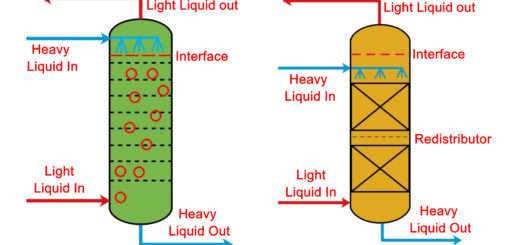
Leaching Process

Leaching Process:- Leaching is a mass transfer operation in which we have a solid material which either contains components which are valuable to us or components which are considered an impurity of the solid, no matter what the case, such components are called solute. We take a liquid which is called a solvent and contact it intimately with the solid in order to extract the solute from the solid and bring it into the liquid thus effecting a separation. Leaching is a solid-liquid extraction process.
The process of leaching generally concerns with processes where the solid is inert and contains soluble solute which is extracted from the inert solid with the help of chemical reaction; for example leaching of valuable metals from waste materials by using sulphuric acid. The process of leaching is extremely common in metallurgical industries.
Factors which affect the Leaching process
- Size of the inert solid: If the inert solid is finely crushed and is used in the process in the form of flakes then the solute will not have much trouble in diffusing through the solid in order to get dissolved in the liquid. If the inert solids are coarse in nature then the soluble solute will face much resistance while diffusing through the interiors of the solid in order to get dissolved in the bulk of the liquid.
- Porosity of the inert solid: An inert solid which is highly porous in nature offers lesser resistance for the solute present in the interior of the solid to transport and get dissolved in the liquid compared to solid which has very less porosity or is non-porous. In non-porous solids, the solute present on the surface of the solid gets dissolved first and then the solute present in the interior of the solid have to diffuse through the layers of resistances offered by the solid in order to reach the liquid. It makes the process slower.
- Solvent: The liquid used should be highly selective towards the solute when it comes to the dissolution process. The nature of the liquid should be such that it should not react or dissolve the carrier solid. It should have low viscosity in order for the solute to diffuse relatively easily. It should be quite volatile in nature because it becomes easier to separate any entrained inert solid from the liquid using flash vaporization process.
- Temperature: The solubility of the solute in the solvent increases with an increase in temperature. The mobility or diffusivity of the solute in the solvent increases with increase in temperature. The viscosity of the liquid decreases with an increase in temperature, this lowered viscosity helps in increased mobility of the solute through the liquid or the solvent.
- Agitation: The general effect of agitating the solid-liquid solution is that the solid-liquid mass transfer coefficient increases with an increase in agitation but care should be taken that the inert solid doesn’t get disintegrated. If most of the mass transfer resistance lies in the interior of the solid then agitation will not have much effect on the leaching process.
Solid-Liquid Equilibrium Diagrams
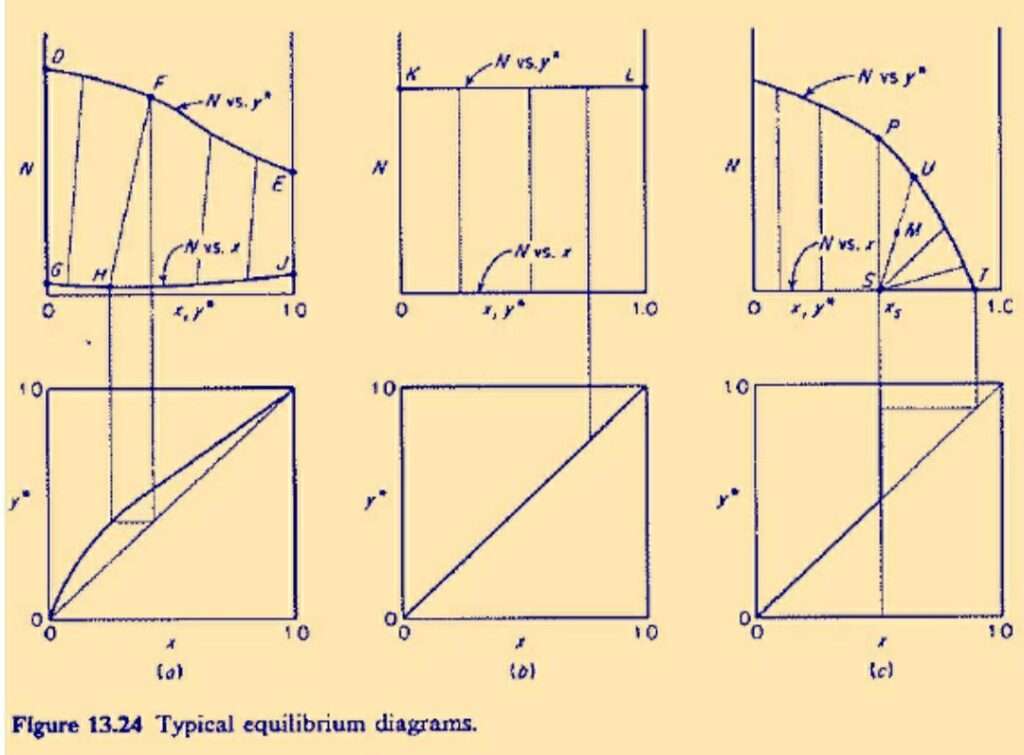
The figure shows three types of cases which are represented on the plot. Here,
- N is the mass ratio of insoluble solid with respect to the sum of the mass of solvent and solute.
- x is the weight fraction of the solute in the effluent solution.
- y is the weight fraction of the solute in the solids or slurry.
Figure (a) represents the case where the solute is infinitely soluble in the solvent. Hence both the weight fractions have the values which range from 0 to 1.
example of such a system is soyabean oil-soyabean mean-hexane. In this figure the curve GHJ is above the N=0 axis, it means the solid is partially soluble in the solvent. Line FH is a tie line and it is not vertical in this case.
Figure (b) represents the case of constant underflow. In this case the weight fractions of the solute associated with the withdrawn solution and the solid have the same value. All the tie lines in this case are vertical.
Figure (c) represents a case where the solute has limited solubility in the solvent. Point T represents the composition of pure solid solute after drainage of the solution.