Absorption vs Adsorption

Absorption vs Adsorption:- Absorption and Adsorption both are one of the most important mass transfer processes used in chemical and process industries. Absorption process and Adsorption process both are called sorption process. A Sorption is a physical or a chemical process by which one substance becomes attached to another substance. Another sorption process is Ion Exchange process.
Definition
Absorption is a process in which a component or components from a gas phase transfer into a liquid phase when the gas phase and liquid phase are brought into contact. The components move to the bulk of the liquid phase hence it absorption is a bulk phenomenon.
Adsorption is a process in which a component or components from a gas phase or a liquid phase are attached to the surface of a solid phase when the gas phase or the liquid phase is brought in contact to the solid phase. Adsorption is a surface phenomenon.
Heat Effects
Absorption is an Endothermic process, it means when the gas component gets absorbed in the liquid phase the thermal energy gets absorbed from surrounding to the system.
Adsorption is an Exothermic process, it means when the gas or liquid component gets adsorbed on the solid phase the thermal energy gets released from the system to the surrounding.
Effect of Temperature of Absorbent or Adsorbent
In absorption process, the absorbent is the liquid phase. When the temperature of the liquid is increased then the dissolved gas components in the liquid gets removed from the liquid. When the temperature is reduced the amount of gas components dissolved in liquid can be increased.
In adsorption process, the adsorbent is the solid phase. When the temperature is increased the extent of adsorption decreases, it means the amount attached to the surface of the solid will decrease. Conversely it also means that the extent of adsorption increases when the temperature is reduced.
Effect of Pressure of the Gas phase
In absorption process; when the pressure of the gas phase increases and the temperature remains constant it means the amount of the component(s) have increased in the gas phase. Large presence of the component(s) in the gas phase means more amounts will tend to get dissolved in the liquid. This also means that if the pressure is reduced then extent of gas components dissolved in the liquid will be relatively lesser.
In adsorption process; there are a limited number of active sites. If the pressure of the gas phase is increased then it means amount of the adsorbate gets increased in the gas phase. It means initially the rate of adsorption will get increased until a point is reached when all the active sites are filled then the rate of adsorption becomes independent of the pressure.
Types: Physical and Chemical
For absorption process; in physical absorption process no chemical reaction takes place between the component(s) present in the gas phase and the liquid phase. The component(s) are transported from the bulk of gas phase to the interface of the liquid phase and then to the bulk of the liquid phase.
In chemical absorption process the component(s) of interest are of such nature that they react chemically with the solvent (liquid). The chemical reaction may occur near the interface or in the bulk region of the liquid. The chemical rearrangement causes a new compound to form in the liquid. This is especially helpful if there are interested in purifying the gas phase then the impurities can be reacted chemically with a solvent.
For adsorption process; in physical adsorption the solid phase is porous in nature and contains potential sites for adsorption. The component(s) from gas phase or a liquid phase directly get attached to the active sites on the surface of the solid phase without undergoing any change in their structure.
In chemical adsorption, when a component to be adsorbed nears the active sites on the surface of the adsorbent, chemical reaction happens which causes the bonds to break thus changes in structure of the component takes place. Chemical adsorption is also called chemisorptions and physical adsorption is also called physisorption.
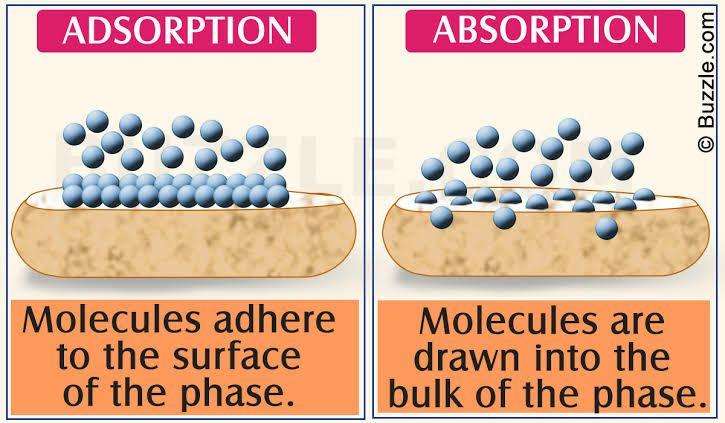
Image:- buzzle