Catalyst Types and Loading Methods
Catalyst Types and Loading Methods:- Catalysts are materials which are added in a chemical reaction in order to increase the rate or the speed at which the reaction is occurring. Every reaction proceeds with a path or a mechanism called the reaction mechanism. Every reaction mechanism has a particular activation energy associated with it. The activation energy of a chemical reaction is the minimum amounts of energy required to take the reactants to the condition in which they will start reacting with each other hence carry out a chemical reaction. If this amount of energy is not available then no chemical reaction will happen. Introduction of catalysts in a chemical reaction results in a different path or a different mechanism whose associated activation energy is much smaller than that of the path or the mechanism without the catalyst.
When a catalyst is introduced in a chemical reaction, the reaction proceeds with both the path but majority of reactants follow the path which corresponds to lower activation energy. The complete amount of catalyst used during the reaction is recovered at the end of the reaction after the reaction gets completed. The catalysts can be of same phase as the reactant, the reaction process is then called homogeneous catalysis. If the catalysts are of different phase than the reactants then the reaction process is called heterogeneous catalysis.
Types of Catalysts
Homogeneous Catalysts:
The catalysts belong to the same phase as that of the reactants due to which when the catalyst is introduced in the chemical reaction it mixes very easily with the reactants due to which the interaction between them leads to high selectivity and high activity. The reaction mechanism of such catalysts is generally well understood. The catalyst regeneration is generally difficult because the separation of constituents is itself difficult.
Heterogeneous Catalyst:
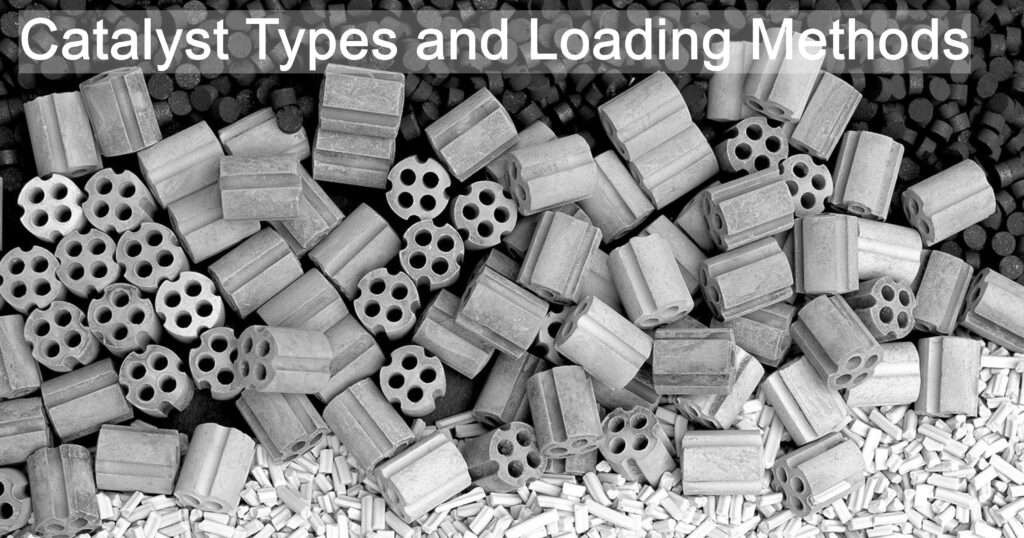
They catalysts do not belong to the same phase as that of the reactants. They are the most common type of catalysts used in industries because when they are introduced in a chemical reaction it is much easier to separate them and recover the products and the catalysts as well. They are available in powdered form, in granules and also supported on carriers. The benefit of using the catalysts in powdered form is that large surface area is available for reaction. The disadvantage is that smaller particles tend to agglomerate due to which they lose their catalytic tendencies. It can be solved if the catalysts are dispersed onto the carriers. They have relatively low activity and selectivity. The underlying reaction mechanism is not well understood.
Bio catalysts:
The natural biological catalysts such as enzymes and nucleic acids are used in industries to carry out chemical reactions. They are gaining popularity over conventional catalysts in industries because of high activity, high reactivity and the easiness in separating products and also the catalyst regeneration is optional in nature.
Catalyst Loading Methods
Vacuum Loading:
The catalysts are first put in the hopper and then they are pneumatically conveyed inside the reactor.
Loading directly from Big Bags:
The catalysts are placed in bags and the bags are lifted with the help of cranes. They are made to pass through dust containment system before they are dropped in reactor.
Dense Loading:
A device is used to spread the catalyst evenly in the reactor in order to reduce void space and to reduce channelling. Either rotating equipment or a propellant can be used.
Sock Loading:
A discharge pipe is connected to the nozzle of hopper and a flexible sock is attached to other end of the pipe. The sock ensures that the pipe is always full of catalysts.
Tube Reactor Loading:
The tubes in the reactor are directly filled with catalysts using manpower. It is a labour intensive job.
Catalyst Types and Loading Methods
image:- matthey