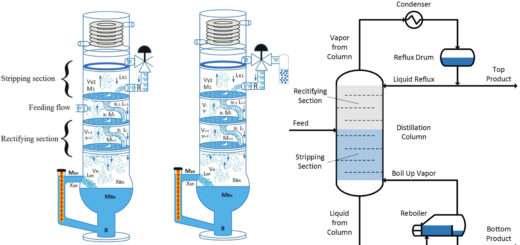
Cryogenic Distillation of Air

What is Cryogenic Distillation ?
Cryogenic Distillation is the process in which Nitrogen and Oxygen are separated from air. In some cases Argon is also separated. The word ‘Cryogenic’ is related to low temperatures and the word ‘Distillation’ is related to separation of constituents from a mixture by utilizing the property of boiling point of the constituents. Thus in cryogenic distillations the components having very low boiling points are distilled selectively at low temperatures. This method produces products of high purity but also it is quite energy intensive. This method of separation was developed by Carl Von Linde in 1895 and it was applied to industries for first time in 1902.
The distillation columns and heat exchangers which operate on very low temperatures are installed inside a very large insulated vessel called the cold box. The refrigeration cycle operates on Joule Thomson effect also called throttling effect. During throttling the gas is passed through an insulated valve or an insulated porous plug, the temperature of the gas changes due to change in pressure.
Cryogenic Distillation of Air

Steps in Cryogenic Distillation of Air
- Pre-treatment, Compressing and Cooling of incoming Air.
- Removal of Carbon Dioxide.
- Heat transfer to bring air feed to cryogenic temperature.
- Distillation of Air.
1. Pre-treatment, Compressing and Cooling of incoming Air
Pre-treatment of Air consists of removal of dirt using filtration devices. Then it is compressed and passed through several stages of intercoolers where the air is cooled in order to remove water vapour.
The temperature of the air has to be brought considerably down to the optimum temperatures suitable for downstream equipments hence often the air has to be further cooled with mechanical refrigeration cycle.
Much of the water vapour gets removed in this step.
2. Removal of Carbon Dioxide
It is important to remove Water vapour and Carbon Dioxide before transferring the air to downstream units because at low temperatures they will freeze and clog the equipments.
The pre-treated, compressed and cooled air is then passed through the molecular sieves. The molecular sieves are porous materials having very small sized pores; the size of the pores is in the dimension of the size of molecules. Molecules whose size is smaller than the pores will pass through the pores and molecules whose size is larger than the pores will not pass through. The materials can be selectively chosen to adsorb the Carbon Dioxide and the remaining Water vapour.
3. Heat transfer to bring air feed to cryogenic temperature
The incoming air feed is passed through a heat exchanger where the cold product stream and the waste gas stream from the cryogenic distillation units are used to cool the incoming air feed so that it gets to the temperature suitable for downstream units.
4. Distillation of Air
In order to separate Nitrogen usually only one distillation column is needed. If Nitrogen is required in very high degree of purity then two distillation columns are used.
In order to separate Oxygen usually two distillation columns are needed. The first column is maintained at high pressure and the second column is maintained at low pressure. The Oxygen is the bottom product in both the columns and Nitrogen is the top product.
The Argon’s boiling point is closer to that of Oxygen hence often in two column distillation process it stays with Oxygen due to which the purity of Oxygen gets reduced. In order to remove the Argon a side stream is drawn where the concentration of Argon is the highest. This Argon can then be further separated from Nitrogen and Oxygen to get Pure Argon.
Image source:- https://www.orthodyne.be , https://www.linde-engineering.com
Content Source:- WIKI