Sulphuric Acid Manufacturing Process
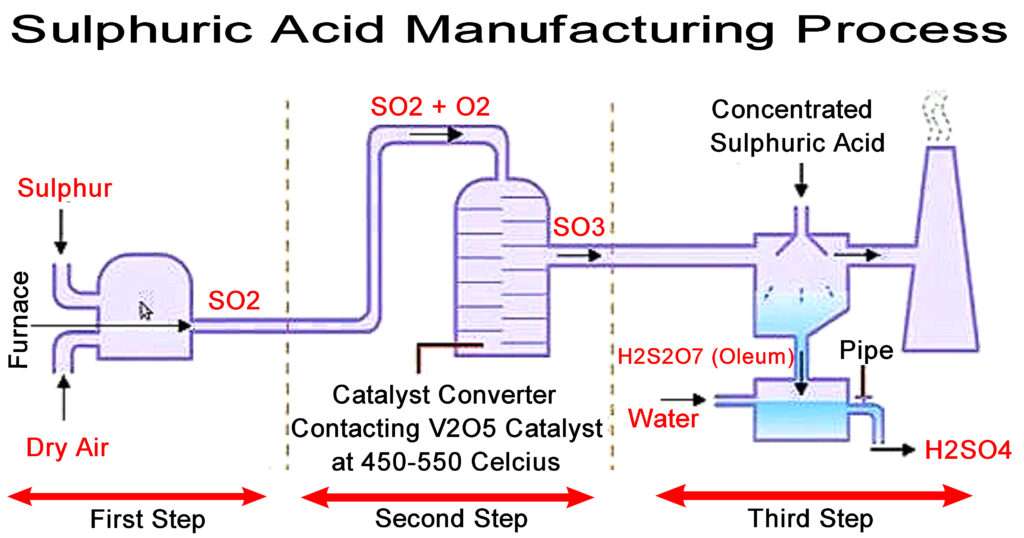
Sulphuric Acid Manufacturing Process
Sulphuric Acid is world’s most produced chemical because of its widespread use in not only in Chemical, Metallurgical, Process, Petrochemical, Fertilizer industries but also in electrical and electronics, Semiconductor industries and also in variety of labs all around the world. The most important use is in phosphate industry and also in metal extraction industries. They also find important uses in Paint and Pigment, Explosive, Detergent, Plastic and Synthetic Fiber industries.
Sulphuric Acid is a colourless liquid which has an appearance similar to that of water. It has a molecular weight of about 98. It is heavier than water. The sulphuric acid can be diluted by water to get acids in various strengths for different purposes. During the mixing process, sulphuric acid should be added to water.
Reason being that since sulphuric acid is heavier than water it will move to the bottom due to gravity and the mixing will be much uniform. In case we add water to sulphuric acid, since the water is lighter than sulphuric acid, it will stay at the top.
Hence, we will have water in the top layer and sulphuric acid in the bottom layer. At the interface the sulphuric acid will absorb water and release heat. This released heat will evaporate a fraction of water near the interface. It will convert it into steam. The steam will expand and the mixture will be splashed everywhere in an exploding fashion.
Almost all the sulphuric acid around the world is manufactured by Contact Process.
Raw Materials used in Contact Process
Sulphuric acid is made up of Sulphur, Oxygen and Hydrogen. So naturally the sources which are rich in these elemental compositions are chosen for the task of production of Sulphuric Acid. The raw materials used can be elemental sulphur by itself or Sulphur Dioxide or Pyrite. Also, air and water are used.
Sulphur can be found from mines, Sulphur and Sulphur Dioxide can be found from stack gases which are obtained from coal or oil industries. Sulphur or Hydrogen Sulphide can be obtained from petroleum desulphurisation. Sulphur dioxide can be obtained from smelting of metal silicide ores or it can be isolated from pyrite.
Sulphuric Acid Manufacturing Process
- Production of Sulpur Dioxide Gas (SO2)
- Production of Sulpur Trioxide Gas (SO3)
- Production of Sulphuric Acid (H2SO4)
Sulphuric Acid Manufacturing Process Steps
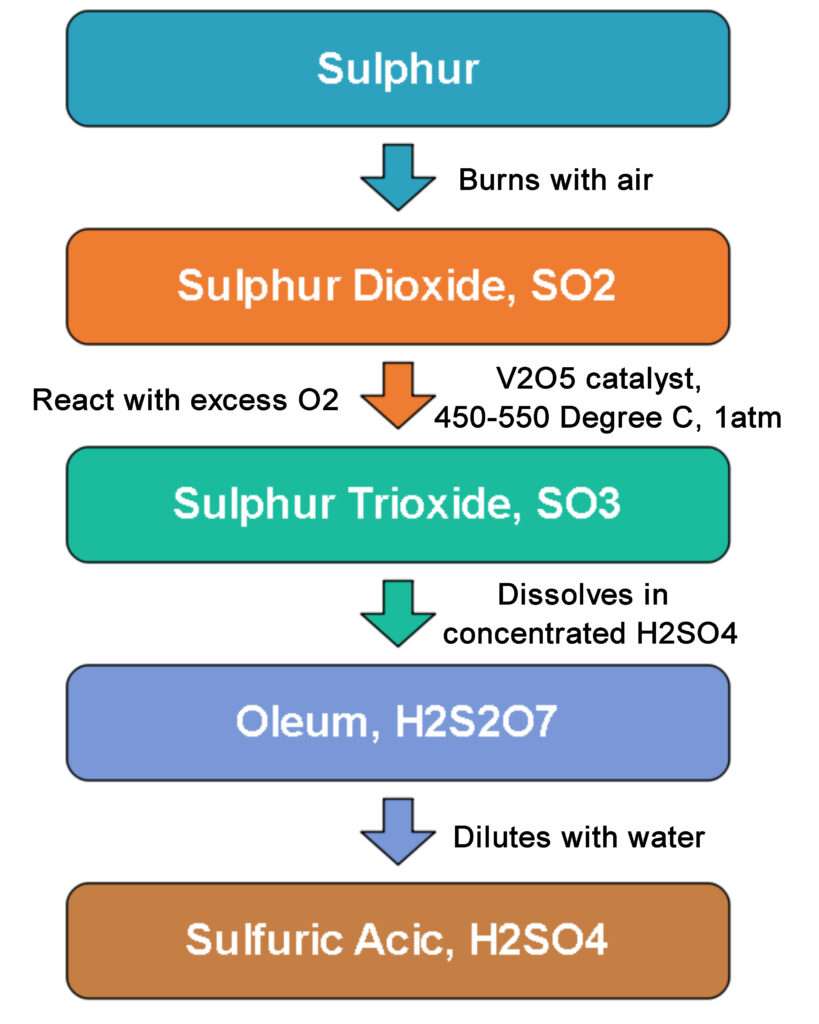
The contact process involves three main steps. First step is oxidation of sulphur to sulphur dioxide. Second step is catalytic oxidation of sulphur dioxide to sulphur trioxide. Third step is hydration of sulphur trioxide to form Sulphuric Acid.
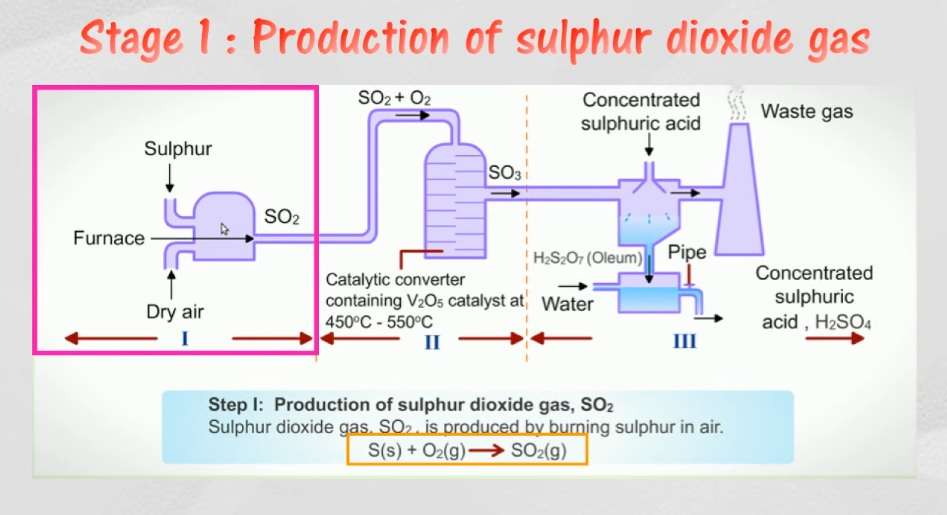
First step: Production of Sulpur Dioxide Gas (SO2)
The sulphur obtained from any kind of source is burned to form sulphur dioxide gas. Care is to be taken in this step so that the air supplied for combustion process is dry. Any traces of water present will result in the formation of acid and cause corrosion within the combustion equipment. Also if the sulphur contains carbonaceous impurities then it has to be filtered. If the gas is not filtered for the impurities then they will be carried over through the equipments and if they reach catalysts then they will tend to deactivate it. The oxidation of sulphur to sulphur dioxide is a highly irreversible process.
Second step: Production of Sulpur Trioxide Gas (SO3)
The catalyst used for the catalytic oxidation of sulphur dioxide to sulphur trioxide is Vanadium Pentoxide. Before sending the sulphur dioxide to the reactor the gas is pretreated to remove any impurities if present by Wet Scrubbing and if there are any fine particles present then the gas is passed through electrostatic precipitator. The oxidation of sulphur dioxide to sulphur trioxide is a reversible process. The pressure is maintained at 1.2-1.5 atm and temperature at 410-430°C.
If the temperature rises above 430°C then the equilibrium is displaced away from Sulphur Trioxide, hence it is very important that operating temperature should be maintained in this range. Commonly the catalytic reactor is designed as a four stage bed reactor. When the gas is passed to one catalyst bed it’s temperature rises from the range of 410-430°C to about 600°C hence after each stage the gas has to be cooled to the optimum temperature again before passing it to the next stage.
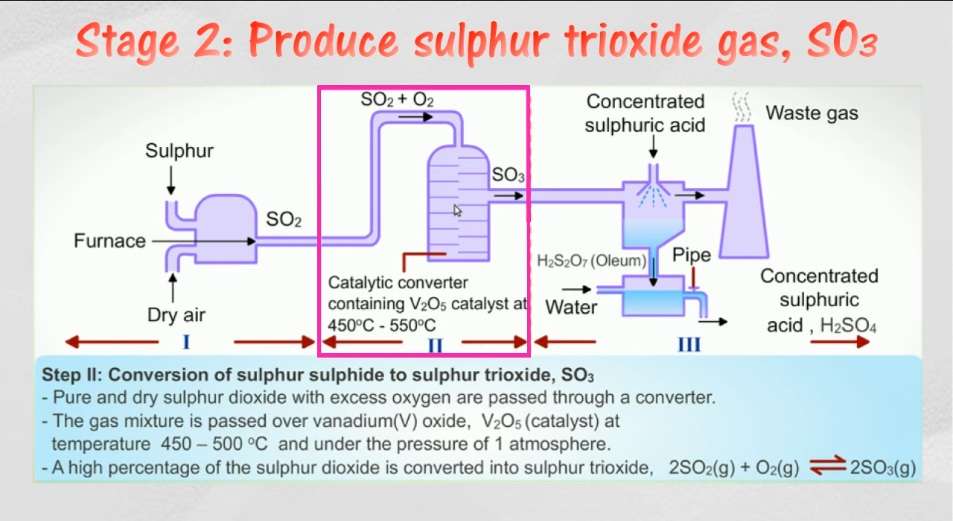
It is important that the sulphur dioxide be prevented from leaking in the atmosphere as they not only pollute the air but also are a major constitute in the cause of Acid Rain. Double Absorption helps in ensuring that more of sulphur dioxide is converted to sulphur trioxide and also overall doing this is beneficial for the process also in terms of purity. After three stages the purity becomes 97-98% and after four stages the purity becomes 99.5-99.8%.
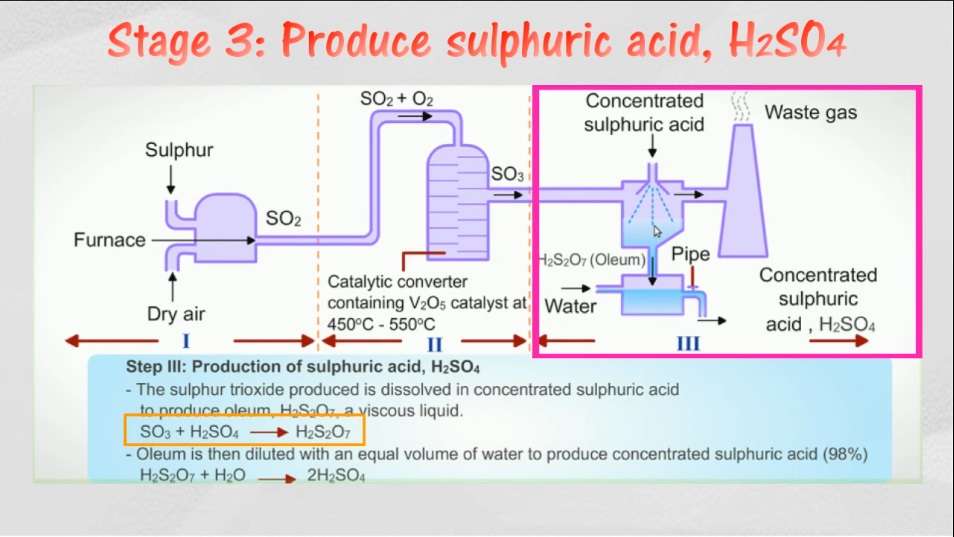
Third step: Production of Sulphuric Acid (H2SO4)
The sulphur trioxide is hydrated by absorption in a packed tower which is filled with Sulphuric Acid of a specific range. This specific range is 98-99%. If this concentration happens to be on lower side then sulphur trioxide is not properly absorbed and forms a troublesome mist. If the concentration happens to be on the higher side then vapours become very significant inside the tower. Hence it is important to maintain a good operation concentration. The resulting acid strength can be adjusted by controlling the flow rates of sulphur trioxide and water. The resulting acid strength can be varied from 91 to about 100% sulphuric acid.
Reference/Image:- Youtube