(NFPA) National Fire Protection Association
National Fire Protection Association (NFPA) is a US-based international nonprofit organization devoted to eliminating death, injury, property, and economic loss due to fire, electrical, and related hazards. NFPA-704 is a standard system for the identification of the hazards of materials for emergency response.
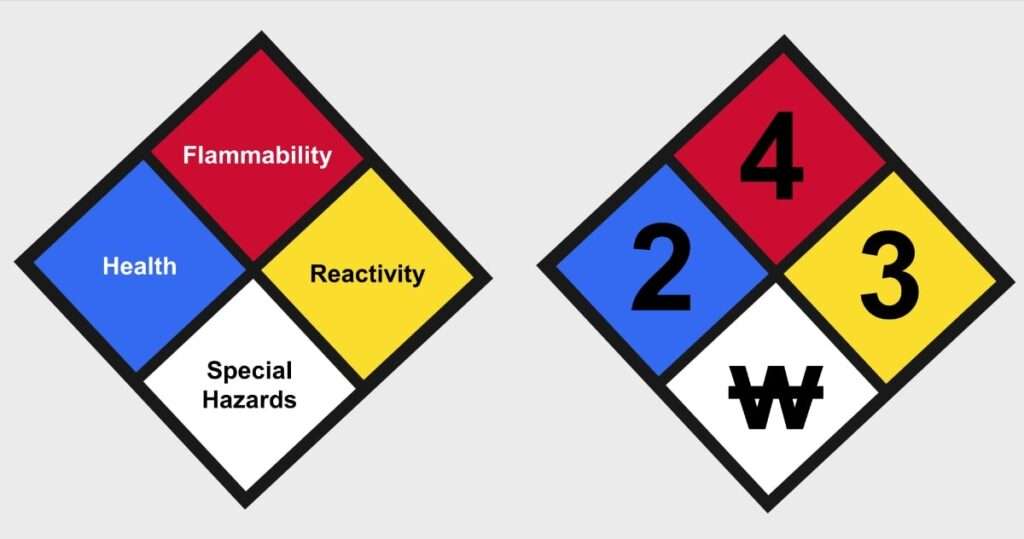
The NFPA-704 defines diagrammatically what is conventionally known as fire diamond or safety square. The diagram helps personals to reliably and quickly identify the risks associated with hazardous materials so that the personals would know how to properly handle the material and also in case of an emergency what procedure should be followed and what measures should be taken.
The square or diamond diagram is divided into four parts. The parts can be seen to have an upper part, a lower part, a left part, and a right part. The upper part is coloured in red, the left part in blue, the right part in yellow, and the lower part in white. The red colour gives information about flammability, the blue colour gives information about health hazard, the yellow colour tells about hazard due to reactivity, and the white is reserved for special hazards. Each part is rated on a scale of 0-4. The lowest number on scale, zero, means no-hazard and the highest number on scale, four, means severe-hazard. The number is assigned only for the red, blue, and yellow part. In case of white part, a symbol is used. Some symbols have been assigned by NFPA but non-standard symbols may also be used if the authority having jurisdiction allows it.
NFPA Symbology
Flammability [Red]:
- 0: The substance/material is non-combustible. Basically it contains those materials which will not burn in air when exposed to temperature of 820°C for a period of 5 minutes. Some examples are, concrete, stone, and sand.
- 1: Those materials whose flashpoint is at or above 93.3°C. These materials require certain preheating before ignition occurs. Example, ammonia, mineral oil, etc.
- 2: Those materials whose flashpoint lies between the range of 37.8-93.3°C. They need moderate preheating before combustion can occur. Example, sulfur, diesel fuel, etc.
- 3: Those materials whose flashpoint lies between the range of 22.8-37.8°C. Example, acetone, and gasoline.
- 4: Those materials whose flashpoint is below 22.8°C. These are those materials which burn quite easily. Example, propane, hydrogen gas, acetylene, etc.
Health [Blue]:
- 0: These are those materials which does not pose any health hazard eventhough they may be flammable. Example, paper, wood, etc.
- 1: These are those materials whose exposure can only cause irritation. Example, acetone, potassium chloride, etc.
- 2: These are those substances whose continuous long-exposure may cause temporary incapacitation and possible residual injury. Example, diethyl ether, ammonium phosphate, etc.
- 3: These are those substances whose short-exposure may cause serious temporary or moderate injury. Example, liquid hydrogen, sulfuric acid, calcium hypo-chloritez etc.
- 4: These are those substances whose very-short-exposure may cause death or major residual injury. Examples, dibornae, phosgene, methyl isocyanate, etc.
Reactivity [Yellow]:
- 0: Those substances which are stable even during fire hazard. Examples, helium, nitrogen, etc.
- 1: Those substances which are normally stable but may become unstable and reactive at elevated temperatures and pressures. Example, propene.
- 2: These are those substances which undergo violent chemical changes at elevated temperatures and pressures, and those that react violently with water. Example, sodium, potassium, etc.
- 3: Those materials which are capable of detonation or explosion but they require strong initiating source. Example, hydrogen peroxide, caesium, etc.
- 4: Those substances which are capable of detonation even at normal temperature and pressure. Example, nitroglycerin, azidoazide azide, TNT.
Special Hazards [White]:
(A) Standard symbols:
- OX: Those substances which are oxidisers. Example, potassium perchlorate, ammonium nitrate, etc.
W: Reacts with water in an unusual or dangerous manner. Example, sulfuric acid, caesium, etc.
- SA: This symbol refers to simple asphyxiant gases such as helium, argon, etc. It is also used when large quantities of dry ice is stored somewhere.
Non-standard symbols:
- ACID: Refers to acidic substances.
- ALK: Refers to alkaline substances.
- COR: Corrosive substances.
- POI: Poisonous substances.
- BIO: Biological hazard.
- RAD: Radioactive.
- CRYO: Cryogenic substances.
Reference:- Wiki