Difference Between Osmosis and Diffusion
Difference Between Osmosis and Diffusion
Difference Between Osmosis and Diffusion
Diffusion:
Diffusion is defined as the net movement of components from higher concentration region to the lower concentration region at the given condition parameters such as Pressure & Temperature. Diffusion process utilizes concentration gradient ( concentration difference) as the major deriving force. Diffusion process can be performed naturally or by means of external force.
Properties of Mass transfer Operation:
- Diffusion process which plays important role in successive distillation process operation, distillation helps to diffusion of the component which is highly volatile to vapor phase region, whereas the low volatile region to the vapour to liquid phase.
- Gas absorption process: gas absorption operation in which solute gas is diffused from the gas to the Liquid by passing through the interface level as well.
- Crystallization operation: Process in which solid crystal particle diffused away from the mother liquor solution to crystal & then deposition of solid surface.
Examples of diffusion process:
- Crystal formed potassium permanganate which got diffused into the water solution & turns the color to blue.
- Molecules of perfumes which is packed inside the spray bottle is diffused into the air & reaches to nose via odour of the perfume.
Types of Diffusion process:
generally there are two types of Diffusion process are described below:
- Molecular diffusion: diffusion of molecule is due to their thermal energy. Rate of diffusion is very slow in this mode. It can be explained by kinetic theory.
- Rate of diffusion. = Total distance travelled / unit time
- Ways to increase molecular diffusion: increase in temperature cause the high motion in molecules with each other so does the rate of diffusion increases.
- Reducing the pressure which reduce the no of collision of the molecules with each other & increase net free mean path by which diffusion rate gets higher.
- Eddy diffusion: Eddy diffusion can be simply understand by the example mentioned below:
Rate of evaporation of water at 25 degree Celsius in complete vacuum is found about 3.3 Kg/m2S of water surface but if we place a stagnant layer of Air at 1 atmospheric pressure & 0.01 m thickness above the water surface the diffusion rate would gets lower by 600 times.
Actually molecular diffusion is Eddy diffusion in following manner:
Take a container which height is about 1.5 m which is filled by the lower half with salt solution & upper half by the water solution & kept it undisturbed, from the data calculation sallt concentration will reach at the top of container about 87% in 10 years, while for reaching it about 99% it will take about 30 years.
Optional or alternate modes:
if we places stirrer into the solution the container & running at 2o RPM which takes about 10 minutes to reach at the top of layer of salt concentration.
Osmosis:
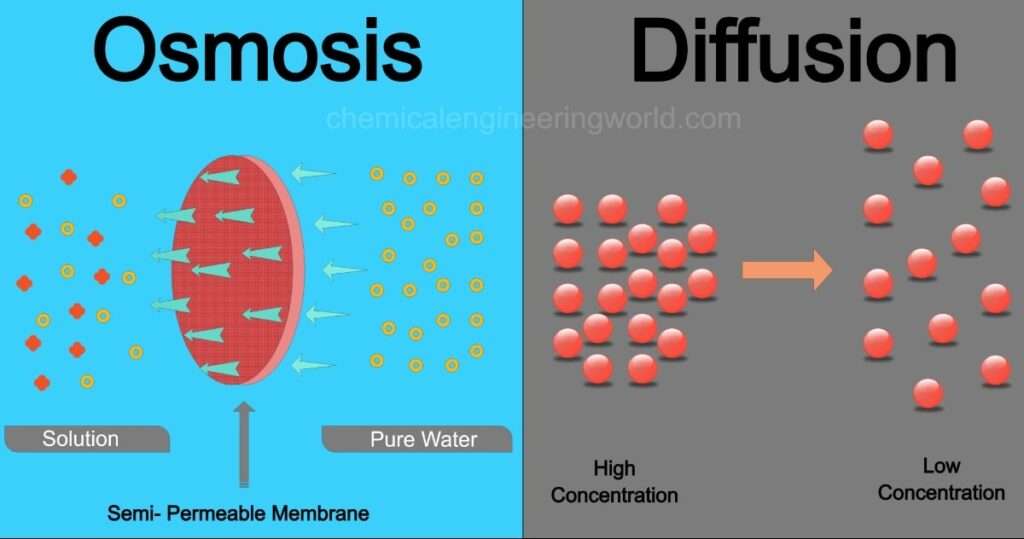
Osmosis is process in which gives pathways or space or move up of solvent molecules by the means of permeable membrane. Osmosis principally involves of movement of molecules from lower to higher concentration regions. Solvent part of solution is moved to the solute part in an Osmosis operation.
Osmosis can be performed in biological system, biological system uses the semi- permeable membrane for the osmosis process. Feasibility of the permeable impermeable membrane is purely dependent over the various factors which are following —–size of molecules – for larger size of molecules or polar molecules is impermeable, for molecules of smaller size it becomes permeable. Other factors on which permeability of membrane is depends solubility, solute size & charge or chemistry.
Concept of osmosis
Osmosis operation carried out when membrane that is partially permeable, used membrane may be cell type which is induced or held beneath the water surface (under the water level) for movement of water molecules from low concentration solute region to high concentration solute region to complete successive operation.
Case I: Cell membrane contains pure water on the both sides, then these water molecules can 0ass in & out in the each every possible direction.
| Diffusion | Osmosis |
| Diffusion doesn’t requires the permeable membrane for operation. | Osmosis process requires the semi-permeable membrane for the separation. |
| Movement of molecules from the higher concentration region to the lower concentration region | Osmosis process involves the movement of molecules from the low concentration to high concentration region. |
| Movement of solute & solvent molecules is possible in diffusion process | Movement of solvent molecules is applicable in the osmosis process. |
| Diffusion is fast process. | Osmosis process is quite slow in comparison with Diffusion operation. |
| Diffusion can be performed or applicable for solid, liquid & gases. | Osmosis applicable for the solvent part of molecules in solution. |
| Diffusion can be performed in any medium | Osmosis process requires mandatory presence of liquid medium. |
Examples of Osmosis process:
- Process of getting water absorbed by the plant via the root system.
- Absorption of water by the fish through their skins & grill body parts.
Reference:- Byjus, vedantu, majordifferences, unacademy






























