Steam Distillation Principle
Steam Distillation Principle :– In usual distillation process, the mixture of components which we have is miscible with each other. If such a mixture is boiled then the vapour pressure it will produce will be dependent on the composition of the components in the mixture. Whatever is the composition of the components in the mixture, the pressure exerted by the vapours formed must be equal to the pressure subjected to the system, technical term for this is operating pressure.
If the operating pressure is one atmosphere then the system of liquid mixture have to be brought to such a temperature at which enough vapours will be produced to equalize the pressures. If the operating pressure is less than one atmospheric pressure then the temperature which needs to be achieved is relatively smaller, if the operating pressure is more than one atmospheric pressure then the temperature which needs to be achieved is relatively larger.
There are cases where doing this may not be feasible:
- If materials to be separated have very high boiling points then more heat is to be transferred in order to increase its temperature. The process becomes more energy intensive and costs more.
- If we have a mixture in which a component is inflammable if it reaches a particular temperature and if this temperature is smaller than the temperature required to achieve the required pressure then we cannot risk carrying out the process due to safety concerns.
- If we have a mixture in which some components are thermally unstable then increasing the temperature too much can degrade the components and affect their properties.
- If we have a binary mixture in which one component boils at high temperature and other component is non-volatile in nature then the process becomes energy intensive.
These cases are easily tackled using a type of distillation technique called Steam Distillation.
Steam Distillation Process
The central idea of Steam Distillation is that we use steam to contribute for the pressure required to equalize the operating pressure. If this is done then the advantage which we get is that we can maintain the temperature of the system smaller than the temperature which will be required if we do not have steam. In order to make use of steam we need to take care to use it with components which are immiscible with water. When the vapours are condensed and if the liquids formed are immiscible then it is much easier to separate them using a decanter.
Steam Distillation Example

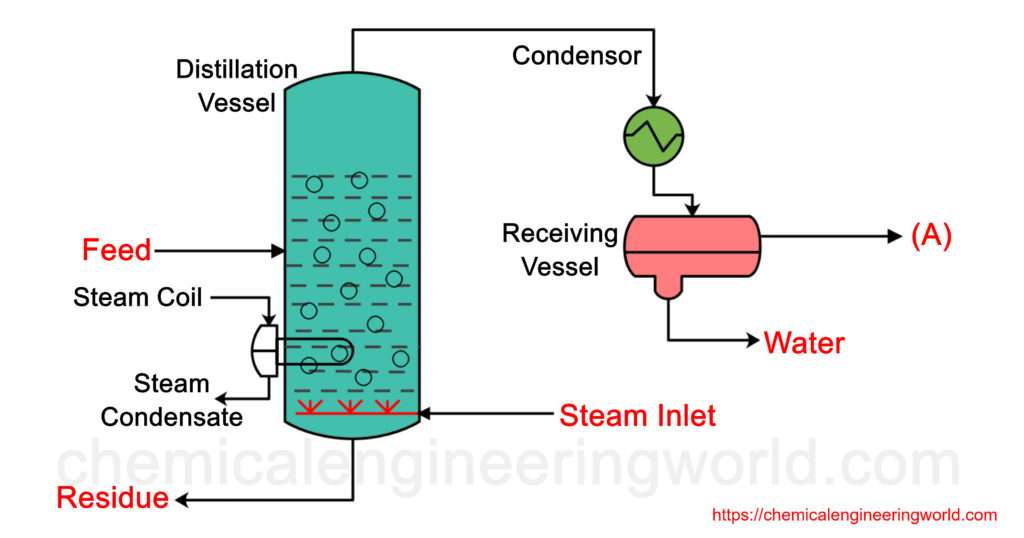
Let’s say we have a binary mixture in which component A is a high boiling component and the other component is a non-volatile component. Suppose A is immiscible with water. We feed the mixture in the column. In the figure, a steam coil is used to increase the temperature of the feed mixture. Another steam line is used which makes the steam to pass through a sparger. Steam passes through the feed mix inside the column and contributes in its share of vapour pressure which when becomes equal to the operating pressure results in the formation of vapours of A at relatively lower temperature. The non volatile component stays in the feed and is removed as residue. Component A along with steam is passed through a condenser where they are condensed and then easily separated.