Liquid-Liquid Extraction
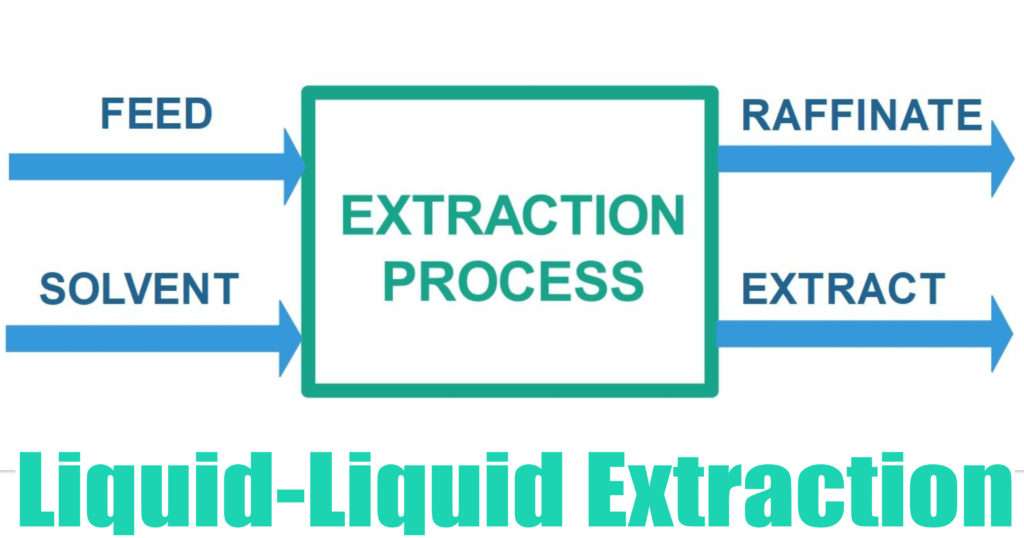
Liquid-Liquid Extraction (LLX) is a mass transfer operation which is used to separate components of a liquid mixture by contacting it with another liquid.
The Feed stream may consist of any number of components. It must have at least two components. Usually, we are interested to separate only one component from a feed stream. This component is called a ‘Solute’. The components other than the Solute are together referred to as carrier liquid.
The Solvent stream is a usually a pure liquid. The selection of the solvent is such that it should selectively dissolve the solute component, which is the component of our interest and must not dissolve other components. If it happens that all components get dissolved in the solvent then choice of solvent should be such that it dissolves more amount of the component of interest relative to other components.

Depending on the amount of feed stream or solvent stream being used, when they are contacted it happens that one of the liquid gets dispersed into the other liquid in form of tiny droplets. The surface tension of the liquids should be such that it must keep them separated after the mixing is done but also not prohibit the movement of tiny droplets into the other phase during the mixing process itself.
Tinier the droplets, faster are the rate of mass transfer of the solute from the feed to the solvent.
If the density difference of both the liquids is not large enough then after the mixing is done both the phases won’t separate easily or instantly and will exist as dispersion, this dispersion is called an Emulsion.
The Emulsion can be broken by working with parameters which affect the density of liquids, such as increasing the Extraction Process Feed Solvent Raffinate Extract temperature of the process or adding another component which helps in separation of the phases. If this is not done then we will require very large extraction equipments which will cost a lot and require large space and this may make the overall process infeasible.
After the mixing process is done, it separates into two phases, the solute rich phase is called an Extract phase and ideally it is made up of Solute and the Solvent. The solute lean phase is called Raffinate phase, it is mostly made up of little bit of solute and the carrier liquid. If the process in not ideal then the raffinate phase may contain little bit of solvent and the extract phase may contain little bit of carrier liquid as well.
Extraction may be a process of choice when:
- Distillation cannot be used if the components to be separated have close boiling point temperatures or the components to be separated are heat sensitive.
- The amount of solute is very less in the feed itself, in essence a dilute liquid mixture.
- We have to separate non-volatile metal solutes in metal extraction industries.
- We have to remove organics from the aqueous stream.