What Is Distillation?

What Is Distillation?
Distillation is a process in which a liquid mixture of volatile components is separated by imparting energy to it in consideration with the boiling points of the components so that selective vaporization takes place. This process can also be used in reverse to selectively condense the vapour mixture.
Suppose we have to boil only one component, one way to do it is to impart energy to it in form of heat at a constant pressure. The temperature of the pure component will continue to increase and when the system reaches a particular temperature corresponding to the pressure called saturation temperature, the vaporisation begins. The temperature of the system remains constant until whole vaporisation is complete.
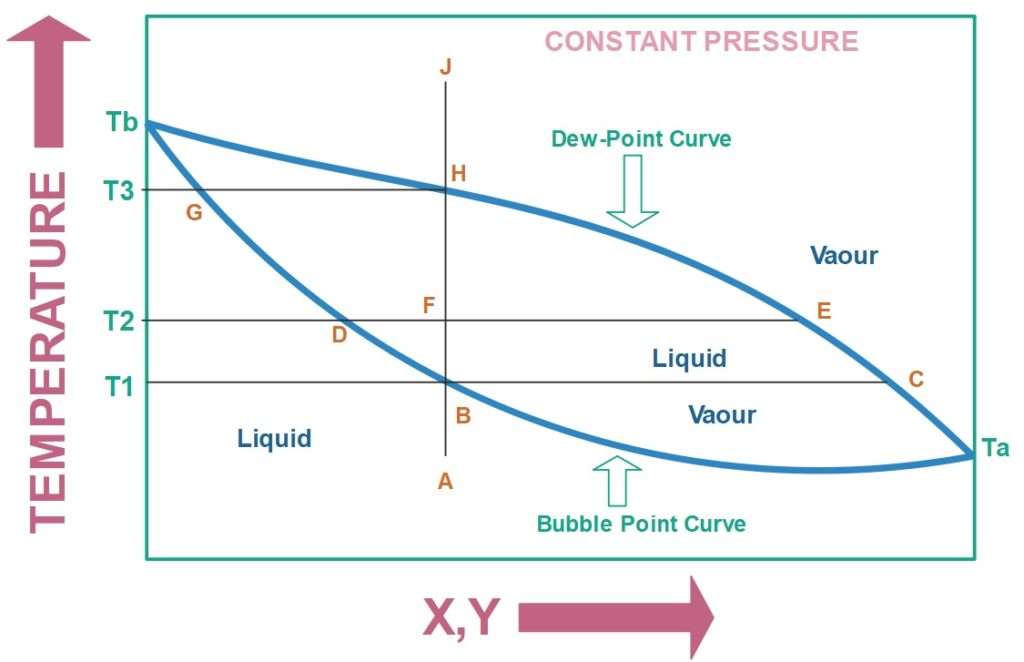
Now suppose we have a liquid mixture of two components. If we attempt to boil the mixture by increasing its temperature at a constant pressure we will find that the liquid mixture does not boil at a single temperature but at a range of temperatures. In the figure the range between B and H corresponds to temperature range of an arbitrary mixture.
When the liquid mixture is vaporized, some fraction exists as a vapour phase and rest of the fraction is liquid phase. Ideally, the composition of vapour phase and liquid phase should be different from each other. The T-xy diagram of a particular liquid mixture tells us the composition of the vapour phase and liquid phase at a particular temperature at constant pressure. If we have a binary mixture then T-xy diagrams are constructed in terms of the more volatile component. T indicates temperature; x indicates liquid phase composition of the more volatile component; y indicates vapour phase composition of the more volatile component.
Suppose we have a mixture whose current state is given by point A, state means the corresponding value of T, x and y. In the T-xy diagram any state which lies below the bubble point curve is completely liquid and any state which lies above the dew point curve is completely a vapour.
If we start heating the liquid mixture, its temperature will keep on increasing; the composition of mixture will stay constant until it reaches the point B. At this point we will obtain a little quantity of vapour. If we draw a constant temperature line T1 passing the point B, it will cut the dew point curve at point C, it gives the vapour phase composition. The liquid phase composition is given by point B.
If we will continue to increase the temperature more and more vapour will form, the system exists in a vapour-liquid equilibrium. Suppose we reach a point T2, The point D gives information of liquid phase composition of more volatile component and point E gives the information of vapour phase composition of more volatile component. The line drawn between point D and point E is called a tie line and point F is called a mixture point.
One important thing to be noted here is that as we continue to increase the temperature, the composition of vapour phase becomes leaner and leaner with respect to the more volatile component.
If we will continue to increase the temperature we will reach point H after some time. This is the point at which last drop of liquid is remaining and rest all have converted to vapour. When this point is crossed the last drop evaporates.
If we will continue to increase the temperature the composition will not change, the composition that this vapour phase has is same as the liquid phase we started with.
Essentially, in distillation we try to capture the vapour when it is richer in more volatile component and then condense the vapour to get a liquid which is richer in more volatile component. Several methods of distillation have been developed to do this properly and efficiently.
The entire process can be done in reverse with a complete vapour phase as well.