Batch Distillation Process
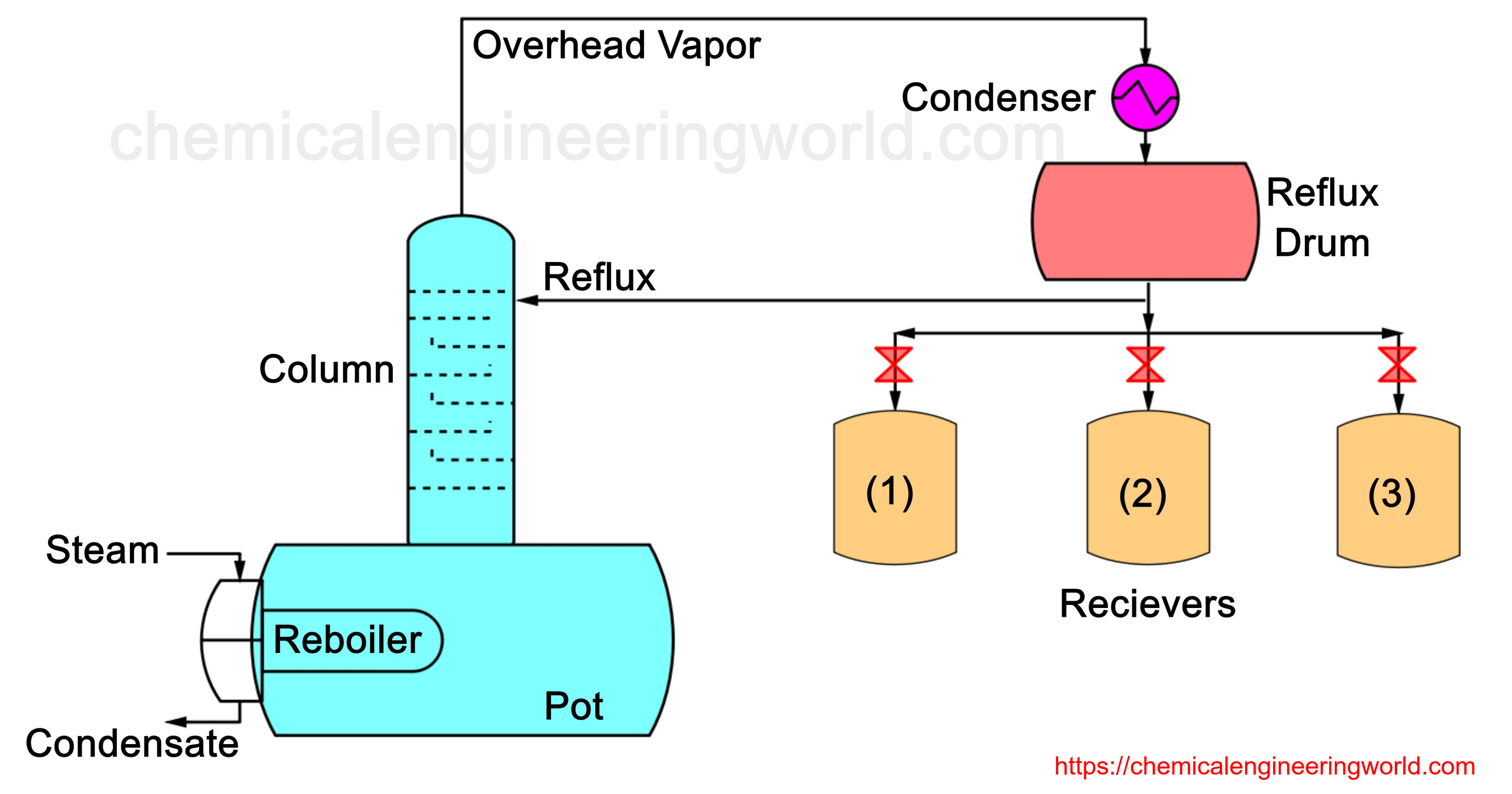
Batch Distillation Process is also called as Differential Distillation or Rayleigh Distillation.
As the name indicates, this type of distillation is a batch process and not a continuous process. The Figure 1 setup consists of a container where a fixed quantity of the liquid mixture is fed initially. This container is called still pot. Energy in the form of heat is input in the system by passing steam through a steam coil. Saturated steam enters at one end and the steam condensate pours out from the other end. A run may last from few hours to several days. Usually it is used when batches have to be completed in eight to sixteen hours.

Figure 2 is called a temperature-composition diagram at a constant pressure of an arbitrary binary liquid mixture. It shows the distribution of components when the mixture exists in a vapour-liquid equilibrium. If the liquid mixture in the still pot has just begun to boil then it will be represented by point B in the Figure 2. The little amount of vapour which will be produced will have a composition corresponding to point C.
If this little amount of vapour will be taken and condensed then the product obtained will be richest in more volatile component. This small amount of product obtained will not be feasible when the overall process is considered hence the liquid mixture in the still pot is allowed to boil further.
If the temperature of the liquid in the still pot increases some more then according to the Figure 2 we enter into the vapour-liquid envelope. It means the material inside the still pot is a vapour liquid mixture at this temperature.
Suppose we increase the temperature to T2, then the liquid phase composition is given by point D and vapour phase composition is given by point E. The composition at point E is smaller than point C it means the more volatile component in the vapour phase has become leaner. The changes are taking place deferentially and the vapour phase compositions are successively averaged with each differential step in boiling.
It is also to be noted that the more volatile component is becoming leaner in the liquid phase and richer in less volatile component as the boiling is continued.
The boiling process is not continued until the point H is reached as the point H indicates that last drop of liquid is remaining. A residual height of liquid mixture is Figure 2: T-xy diagram always maintained in the still pot because boiling it completely will make the composition of vapour same as the liquid composition we started with, initially.
The goal is to get the more volatile component in richer composition in the vapour phase as compared to the liquid phase.
The vapour is passed through a condenser where it is condensed and is collected into a receiver. Usually it happens that many receivers are used during the entire operation with the purpose of collecting more volatile component in different levels of purity referred to as cuts.