Fractional Distillation Process
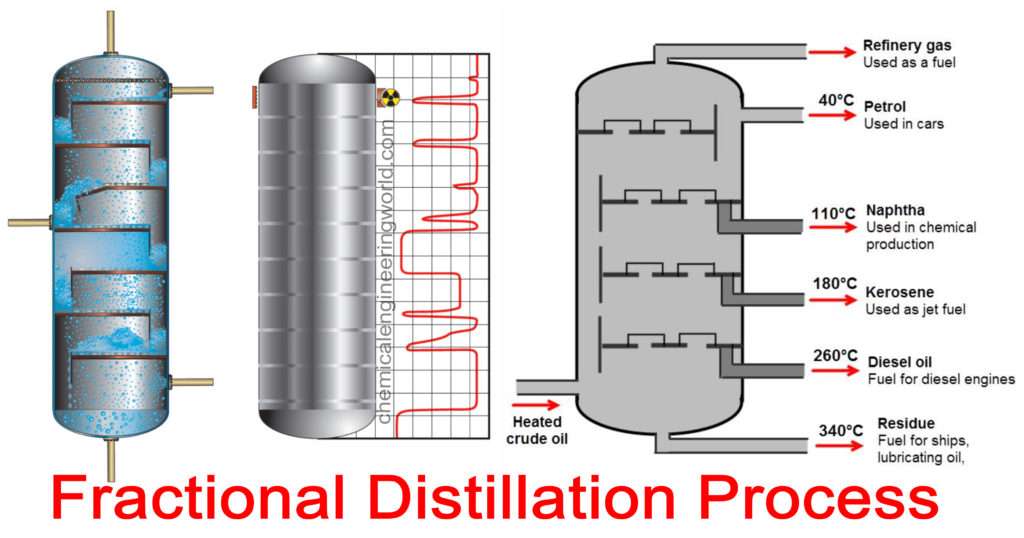
Fractional Distillation Process:- This type of distillation process is usually used industrially to get large amounts of products and also to get products in different-different compositions called fractions.
Principle of Fractional Distillation Process
If we boil a multi-component mixture then those components which have relatively lower boiling points will start to vaporize earlier. If we increase temperature even more then more components will vaporize. The vapours which are formed can have several components and is rich in relatively more volatile components. If the temperature of the vapours is decreased a bit then components whose boiling points corresponds to the temperature will condense.
We will get a condensed liquid mixture of certain composition. If we decrease the temperature even more then those components will condense whose boiling point corresponds to this decreased temperature. In this way we obtained a liquid mixture of different composition. The temperature of the vapour can be continued to decrease and liquid mixtures can be continued to be obtained in various fractions.
Working of Fractional Distillation Process
A distillation tower is a large column consisting of several trays attached to the column. Each tray is separated from the other by a certain distance. Trays are the stages of the distillation process where mass transfer of more volatile components occurs from liquid to vapour phase and the mass transfer of less volatile components occurs from vapour to liquid phase.
The feed is charged in the distillation column at some intermediate distance in the column. A reboiler boils the liquid mixture, the vapour generated by it is directed in the column. The vapour contains various components, each component corresponds to a particular boiling point.
The temperature of the column is highest at the bottom. The temperature decreases if we move from bottom of the column to top of the column. The temperature is lowest at the top of the column. This condition indicates that a temperature gradient exists inside the column.
A fraction of vapour from any part of the column can be drawn and then it can be condensed. Depending on the location from where the vapour is drawn, the composition of the condensate obtained will differ. Vapours from several locations can be drawn simultaneously and they all can be condensed to obtain condensates of differing compositions, they are called fractions.
The fractions obtained from the locations at the top of the column are relatively more volatile or low boiling component. The fractions obtained from near the bottom of the column are relatively more volatile or high boiling component.
Advantages of Fractional Distillation Process
- It is a steady state continuous process hence a large amount of feed can be processed and a large amount of products can be obtained.
- Due to the large number of stages involved and also due to reflux, the product purity obtained is also good.
- A greater degree of separation between products is obtained by taking advantage of multiple vaporization and multiple condensations.